I. Definition of Medical Device
Do you know what a medical device is? A medical device is a device that can be used for any medical purpose. Medical device, generally means equipment, instruments, IVD reagents (in vitro diagnostic reagents) and calibrators, apparatus, materials, and other similar or related items that are used directly or indirectly in the human body, including the required computer software.
Their effectiveness is obtained mainly by physical means etc., not by pharmacological, immunological, or metabolic means. Or these means are involved but only play a supporting role. Medical devices are the most essential elements of medical, scientific research, teaching, institutions, and clinical disciplines’ work. At the same time, medical devices are also the basic condition for the continuous improvement of medical science and technology. It has become one of the important indicators of the modernization of healthcare. The development of the medical field depends a lot on the development of devices. If it can break through the bottleneck, it can play a decisive role in the development of the healthcare industry. Medical devices include professional medical devices and home medical devices.
II. General Intended Purpose of Medical Device
Hospitals and other medical institutions use medical devices mainly to achieve the following purposes
(i) Diagnosis, prevention, monitoring, treatment, or relief of disease.
(ii) Diagnosis, monitoring, treatment, relief, or functional compensation for injury
(iii) Testing, replacement, regulation, or support of physiological structures or processes;
(iv) Life Support;
(v) Conception control;
(vi) Providing information for medical or diagnostic purposes through the examination of samples from human tissues.
III. Medical Device Classification in Different Countries/Regions

In addition to understanding the definition of medical devices, you need to understand the classification levels of these medical devices in different countries. This is very important. Due to the differences in the medical device regulatory legal system in each country / region, the classification management regulatory system that is compatible with it also presents different characteristics. We will introduce the medical device classification and management regulations for the United States, the European Union, Japan, and China.
In addition to understanding the definition of medical devices, you need to understand the classification of these medical devices in different countries. This is very important. Medical devices vary according to their intended purpose and indications. Due to the differences in the legal system of medical device regulation in each country/region, the system of classification and management regulations has different characteristics. We will introduce the medical device classification and management regulations for the United States, the European Union, Japan, and China.
1. United States —- FDA
According to the U.S. Federal Food, Drug, and Cosmetic Act (FD&C Act), the FDA (Food and Drug Administration) classifies medical devices into three classes(Ⅰ, Ⅱ, Ⅲ), which depend on their risk level. Among these 3 classes, Class I means the lowest risk and the highest risk level is Class III. The FDA has clearly defined its product classification and management requirements for each medical device in a catalog of more than 1700 medical devices. Any medical device must first clarify the product classification and management requirements for listing if it wants to enter the U.S. market.
| Class | Risk Level | Regulatory Control | Examples |
| I | Low | General Controls | Electric toothbrush; Tongue depressor; Oxygen mask; Reusable scalpel; Bandages; Hospital bed |
| II | Medium | General Controls + 510(k) Premarket Notification | Hearing aids; contact lenses; medical gloves, sphygmomanometers |
| III | High | General Controls + 510(k) Premarket Notification + Premarket Approval(PMA) | Pacemakers, intrauterine devices, baby incubators, breast implants |
① Class I devices
Devices with little or virtually no risk. Such devices have minimal contact with patients and have minimal impact on their overall health. It can be assured of efficacy and safety with general controls. Devices belonging to this class include, for example, crutches, spectacle lenses, adhesive tape, etc. These devices are the most common class of devices regulated by the FDA, accounting for 47% of the devices approved on the market.
② Class II devices
Products with a certain degree of risk. They are more likely to be in constant contact with the patient. This may include devices that come into contact with the patient’s cardiovascular system or internal organs and diagnostic tools. In addition to the above general controls, these products must also meet the special requirements set by the FDA or other industry-accepted standards. Devices belonging to Class II include medical gloves, electric wheelchairs, hearing aids, diagnostic catheters, etc. FDA has additional mandatory standards for specific products.
③ Class III devices
With greater risk or hazards. In general, Class III devices are the most life-sustaining, life-supporting, or implanted devices that are potentially dangerous to patients and may cause injury or disease. Devices belonging to Class III include pacemakers, intrauterine devices and baby incubators, etc.
2. European Union —- European Parliament and of the Council
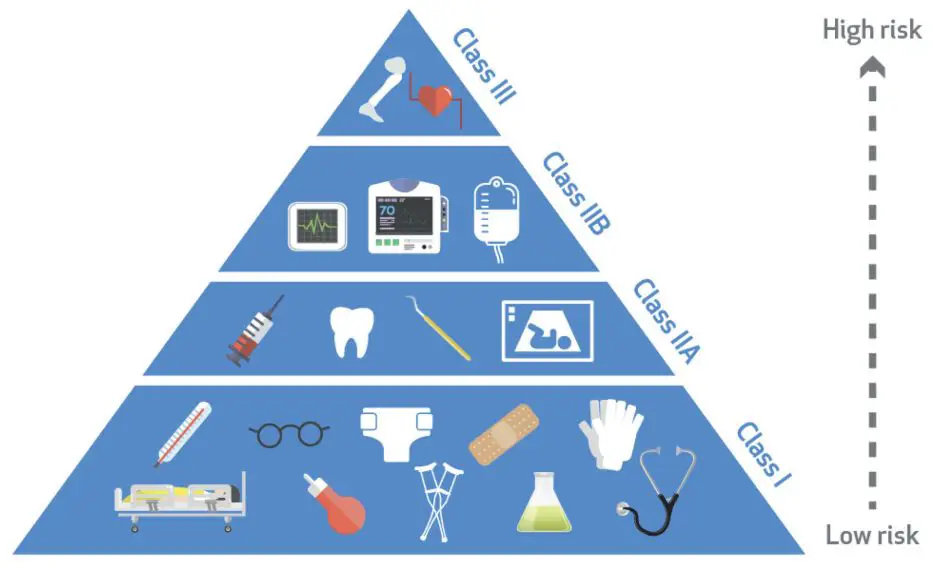
Regulation (EU) 2017/745 (MDR), was officially published by the European Union on May 5, 2017, and entered into force on May 26, 2017. This regulation replaced the previously issued MDD (93/42/EEC) and AIMDD (90/385/EEC) from May 26, 2020. The EU classifies the level of medical device products according to the degree of risk that the design and manufacture of medical devices may pose to the human body. The medical devices are classified into four classes, which rank from low risk to high risk, Class I, Class IIA, Class IIB, and Class III. These four classes overlap and intersect with the classification of non-invasive devices, invasive devices, and active devices. However, here we mainly introduce the rules of classification according to the risk class.
| Class | Risk Level | Categories | Examples |
| I (Class I, Class Is, Class Im, Class Ir) | Low | Measuring device; Sterile; Reusable surgical instrument | Tongue depressor; Oxygen mask; Glasses; Bandage; Sphygmomanometers |
| IIA | Low to Medium | General non-implantable devices; General implantable devices | Surgical gloves, hearing aid; B-mode ultrasound, infusion set |
| IIB | Medium | Implantable devices; Devices specified in MDR Appendix VIII, Article 12 | Sutures; Joint screws |
| III | High | Special non-implantable devices; Special implantable devices; Devices specified in MDR Appendix VIII, Article 21. | Coronary artery stents; heart valves; pacemakers; breast implants |
① Class I (Class I, Class Is, Class Im, Class Ir)
Usually refers to medical devices that come into contact with the human body or only contact with finished skin. There are several subcategories subdivided under this classification. Class Is, refers to medical devices that end up on the market in sterilized form. Class Im, refers to medical devices with measurement functions. Class Ir, refers to reusable surgical devices.
② Class IIA
Generally refers to devices with a higher risk level than Class I Devices. These devices are generally invasive devices for temporary use, active medical devices with energy exchange or measurement.
③ Class IIB
The risk level of Class IIB devices is higher. It generally refers to devices that pose a potential risk to humans or are used for extended periods of time.
④ Class III
Highest risk level. Generally used in the human central circulatory system or brain.
3. Japan —- PMDA
According to Japan’s Medical Device and Pharmaceutical Regulations, medical devices are classified into four classes according to their risk level: Class I, Class II, Class III, and Class IV. Class I is general medical devices. Class II is controlled medical devices. And Class III and Class IV are highly controlled medical devices. A medical device registration certificate is valid for 3 years. The Pharmaceuticals and Medical Devices Agency (PMDA), an independent administrative body in Japan, is the approval authority for medical devices.
Class I medical devices must be submitted to the PMDA pre-marketing instructions before listing. The document does not need to be reviewed and approved by the PMDA. Class II, Class III and Class IV medical devices need to be approved by PDMA before marketing. The medical device marketing license holder must submit a premarket approval application to the PMDA. After the device is approved by the PMDA, it can be marketed.
| Class | Risk Level | Control |
| I | Insignificant | Premarket Notification |
| II | Low | Generally controlled, PMDA Approval |
| III | High Risk on Malfunction | Highly controlled, PMDA Approval |
| IV | High risk and may cause life-threatening | Highly controlled, PMDA Approval |
① Class I medical devices
Belong to the general management of medical devices. The risk of this class to the human body is extremely low. For example: in vitro diagnostic instruments, scalpels, surgical forceps, and X-ray film.
② Class II medical devices
Controlled medical devices. The risk to the human body is low. For example MRI devices, electronic endoscopes, catheters for digestive organs, and ultrasound diagnostic devices.
③ Class III medical devices
Highly managed medical devices. The risk to the human body of device in this class is relatively high. For example dialyzers, artificial bones, and artificial respirators.
④ Class IV medical devices
It is a highly regulated medical device and is highly invasive to the patient. Failure of the device can lead to life-threatening conditions for patients. For example, pacemakers, artificial heart valves, artificial blood vessels, etc.
4. China —- NMPA
In China, medical devices are regulated by the National Medical Products Administration (NMPA). To market a medical device in China, an application needs to be submitted to the NMPA, which will review all applications and has strict requirements for documentation, testing, and clinical data submitted. The classification of medical devices is based on NMPA Decree No. 15 and its classification database. Depending on the risk class, medical devices can be classified as Class I, Class II, and Class III. You may already have some existing test reports, but China still requires domestic testing for Class II and Class III devices. The risk class of medical devices should be determined based on the intended purposes, structural characteristics, use pattern, use status and whether contact with the human body and other factors.
| Class | Risk Level | Control |
| I | Low | General Management, no need for testing reports |
| II | Medium | Full registration dossier and technical review |
| III | High | Full registration dossier and technical review |
① Class I Devices
Class I medical devices belong to the low-risk level. To ensure the safety and effectiveness of the devices, the implementation of routine management is necessary. For example scalpels, surgical scissors, manual hospital beds, medical ice bags, cooling patches, stethoscopes, gauze bandages, etc.
② Class II Devices
Class II devices are relatively high risk. They need strict management to ensure their safety and effectiveness. Class II medical devices belong to a wide range of items, It includes Band-Aids, thermometers, blood glucose meters, oxygenators, nebulizers, etc.
③ Class III Devices
Class III medical devices are devices that require strict management to ensure their safety and effectiveness. Highly advanced technology, advanced risk, and high management are three characteristics of Class III medical devices. The devices include CT machines, medical ventilators, gene sequencers, medical endoscopes, etc.
IV. Classify Medical Devices by End-user
Medical devices are used for a wide range of purposes. Nowadays, user needs for medical devices extend to the environment and conditions in which they are used.
The MedTech industry is also increasingly looking at the end user’s place in product design and development. The easier a medical device is to operate, the easier it is to gain the user’s choice. Whether the end user is a patient, a home caregiver, or a medical professional, they expect medical technology companies to provide more personalized products, simpler procedures, and a better user experience. When classifying medical devices by end-user needs, they can be primarily classified as professional medical devices for hospitals, clinics & ambulatory care centers, and home healthcare medical devices.
1. Professional Medical Devices for Hospitals
The medical devices used in hospitals are the most important. A hospital must have equipment such as defibrillators, patient monitors, surgical tables, ECG machines, anesthesia machines, sterilizers, lights, ultrasound and electrosurgical equipment, and blanket/fluid heaters. These devices allow doctors to make professional diagnoses and treatment plans for patients. Medical devices in hospitals are mainly used to include general surgery, respiratory, neurology, gastrointestinal surgery, cardiovascular, orthopedics, dentistry, diagnostic imaging, etc. The growth of some chronic diseases is also driving the growth in demand for medical equipment, especially diagnostic equipment. Hospitals hold the largest share of the medical equipment market.
Because of the extreme demand for medical devices used in hospitals, the cost of these devices is relatively high. Over the past decade, the medical device industry has made significant developments in the implementation of new technologies. Some advanced devices are equipped with components such as chips, batteries, sensors, and some other accessories that need to be replaced on a regular basis. This also means relatively high acquisition costs and subsequent maintenance costs. The high price of the devices makes them unaffordable for individual users and even some hospitals, thus limiting the growth of the market.
2. Ambulatory Care Medical Device
In addition to hospitals, there are ambulatory care centers that require medical devices. Nowadays, ambulatory care centers can provide a one-stop service for patients, which includes diagnostics and treatment. Ambulatory care will cover a wide range from urgent care to ambulatory surgery to diagnostic imaging. What’s more, it can even include physical therapy, dental clinics, and retail pharmacies.
Due to the variety of patient populations and different care options in outpatient centers, there are challenges in medical equipment planning. Medical devices play an important role in attracting patients to outpatient centers.
3. Home Health Care Medical Device
A home medical device is a medical device provided for use in addition to a professional medical facility. Users can use the device directly or with the assistance of non-professional caregivers or family members for care treatment. In recent years, healthcare has gradually shifted care from the hospital setting to the home environment. Therefore the demand for home medical equipment needed for their care is also increasing.
Home healthcare medical devices offer more comfort and convenience to care recipients or patients than hospital-based care. The doctor will also prescribe the appropriate home medical equipment based on the patient’s needs. Home medical devices may include devices such as glucose meters, heating pads, hospital beds, crutches, CPAP machines, nebulizers, wheelchairs, etc. It may also include monitoring devices such as an Apnea monitor, fitness trackers, ECG monitors, blood pressure cuffs, etc.
Apart from bringing convenience to life, household medical devices also save costs for both the care recipient and the healthcare system. However, because some medical equipment is more complex to operate when the equipment is used under inappropriate conditions or improperly operated by the operator, the effectiveness of the use of the equipment will be affected. This is especially for those devices that require highly for proper operation or for maintenance conditions. Therefore, the production and sales of home medical equipment also have relatively strict requirements.
V. Medical Device Market Size 2022
According to the market research report, the global medical devices market reached USD 532.62 billion in 2021. It is estimated to reach $734.39 billion by 2027, growing at a CAGR of 5.5%. The global medical device market demand is significantly lower than expected due to the Covid-19 pandemic. The number of elective surgeries and emergency room visits to hospitals has decreased significantly. Those less serious or non-life-threatening procedures, such as dental surgery and orthopedic surgery, are down significantly, and so is the demand for medical devices through 2020. However, by 2021, this number has rebounded and the market demand for medical equipment has increased. The medical device market analysis will mainly focus on the device types, regions, and end-users.
By Device Type
The cardiology devices segment is expected to account for the largest share of nearly 19% due to the increased incidence of heart disease. Heart disease and relative health problems are the leading cause of death worldwide. And cardiovascular devices are used to diagnose and treat these diseases. In addition, due to the Covid-19 pandemic, the respiratory segment is estimated to grow at the fastest rate of 7.3%. Under the impact of the epidemic, the need for ventilators for patients has increased significantly. The neurology segment is expected to exceed 4,000 units by 2027.
By Regions
The market size in North America has reached $ 188.14 billion in 2021. North America is expected to hold the largest market share, especially in the U.S., due to the continuous innovation of medical device products in the U.S. and Canada. The U.S. medical device industry is an important part of the healthcare industry. Among the leading market players are Johnson & Johnson, 3M, Abbott Laboratories, and Baxter International. In addition to North America, Asia Pacific is expected to grow fastest in the overall medical device sector.
By End-user
If we analyze the market by end-user, we will find that the hospital segment held the largest market share. This is due to the increasing emphasis on routine diagnosis and timely treatment of patients by hospitals and the government. This also drives the market growth over the forecast period.
Competitive Landscape
The global medical device market is fragmented, but a few companies hold a major share. These include Medtronic Inc., 3M Co., Johnson & Johnson, Abbott Laboratories, Siemens Healthcare, Stryker Corp., and Koninklijke Philips NV. These market players are employing various strategies to maintain their market share, which include product launches, collaborations, mergers and acquisitions, and expansion. For example, Stryker completed the acquisition of US-based medical device company Guass Surgical in 2021.
VI. Further Reading — Importance of Medical Device Safety
We should recognize that how to ensure the safety of medical devices has been a challenging issue in the field of security for a long time.
From thermometers and disposable infusion sets to monitors and ventilators, from pacemakers and artificial blood vessels to intravascular stents and artificial joints, it is clear that most of the medical work in hospitals requires the assistance of medical devices. Not only that, many medical devices will be temporarily or long-term implanted in the human body. For medical personnel, we can not ignore the safety of medical devices. It plays a pivotal role in the physical rehabilitation of patients and the effectiveness of treatment.
If the safety of medical devices is not high enough, it will not only cause economic losses to the manufacturers but also directly endanger the lives of patients. Therefore, governments in different regions have different stringent standards for medical device listing requirements. And this is why medical device compliance is so important.












