Medical & Medical PCB
The development of medical treatment is a microcosm of the development of human society. The human body is fragile, there will be injuries due to external collisions, and there will be sickness due to bacterial and viral infections. Therefore, since the birth of human beings, medical treatment has had a huge market in human society. With the continuous development of society, human’s demand for health and longevity is getting higher and higher, which has brought a strong driving force to the development of medical care. With the development of society, many medical devices are becoming more intelligent. The development of medical PCB is also crucial to the development of medical treatment.
After the industrial society, human cognition has been newly improved, with a deeper understanding of the human body and a higher demand for medical devices. In today’s society, various medical electronic devices emerge in an endless stream, which greatly promotes the success rate of medical care. It can be said that medical electronic devices have made great contributions to medical care. The core component of electronic equipment is the printed circuit board (PCB), and the development of medical PCB has promoted the development of medical care.
The current era we live in is an era of very developed medical technology. Many terminal illnesses that could not be treated before have also found a treatment plan. The fragile body is no longer fragile through modern medical protection, and the damage to the body by collision or germs can also be cured. The development of medical treatment has made human beings farther and farther away from death. Various intractable diseases have been effectively solved, and almost all organ failures can be treated. Doctors are angels and heroes in life, they deserve everyone’s applause, but the development and application of medical devices and medical PCB are also exciting. Behind this, manufacturers of medical electronic equipment and related practitioners have shouldered a very heavy responsibility. They actively develop new medical equipment and maintain the normal operation of medical equipment.
There are many types and ranges of medical electronic devices, and various electronic technologies are accelerating the penetration of the medical industry, from small wearable devices to large whole-body imaging systems. Patient care and monitoring of related physiological indicators are also becoming more and more intelligent. These intelligent devices are inseparable from medical PCB, which are an important part of modern medical care. Whether it is ultrasound equipment or health detection equipment such as CT scans, or physiological index monitoring equipment such as body temperature detection instruments and blood glucose monitors, medical PCB have played a very important role, and their development has greatly reduced the workload of hospital doctors and nurses.
Technology and Healthcare
The development of new technologies always brings social progress. The development of different new technologies promotes the progress of human beings in various fields, which has brought a great impact on the production and life of human beings. However, this is different in the medical field. The introduction of new technologies will be relatively conservative and cautious. The wrong use of technology may bring great suffering to patients. Every medical device is closely related to the health of patients. Different from ordinary PCBs, medical PCB have many related market access systems, and manufacturing medical PCBs also requires higher professionalism.
Nowadays, many medical PCBs are developing in the direction of miniaturization, especially some wearable devices and implanted devices. The size of the PCBs they use is often relatively small, and there is no external power cord. This type of medical device consumes less power and can maintain a long enough life on battery power alone. To accomplish these, the related medical PCBs need to have low resistance and high work efficiency. In addition, many medical PCB are embedded with related wireless information modules to transmit the signals of medical equipment to the hospital.
Above we discussed related medical electronic equipment miniaturization, how small is the smallest medical equipment?
In the medical market, an academic term that has become increasingly popular recently is “miniaturization.” Research on the latest medical devices has reached the micron or even nanoscale, enabling the delivery of drugs through tiny medical electronic devices. This technique is known as “electroceuticals”. Electroceuticals’ PCB board is very small, but it also has IC chips and related sensors embedded on it. This electronic component is small enough that Electroceuticals can swim in blood. The relevant PCB design of Electroceuticals guarantees that it can regulate the delivery of drugs according to the needs of the body. Electroceuticals are cutting-edge medical devices, and many people will benefit from breakthroughs in this technology.
Medical PCB: Rules and Standards
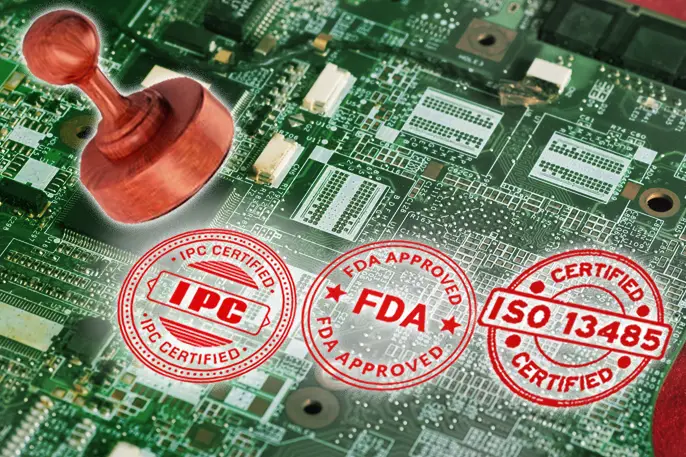
Unlike commonly used consumer electronics, medical PCB have quite a few safety qualities and standards. In hospitals, the accuracy of medical equipment can often affect the judgment of doctors and the treatment plans given by doctors. The treatment and recovery of patients can be affected by the performance of medical equipment, and it can be hard to accept that the performance of a machine may affect one’s own health. But in fact, your worries are superfluous, medical electronics must comply with more and stricter safety and quality standards before they are allowed to be used
There are many rules and standards that medical devices comply with, and even different markets have their own standards. The international mainstream standards include the US Food and Drug Administration (FDA), Federal Communications Commission (FCC), International Standards Organization (ISO) and international standards. Electrotechnical Commission (IEC). Most medical device companies are striving to improve the quality of their products to meet more standards so that products can enter more markets.
Medical standards vary from region to region, and the most widely used textbook on e-health design and development is 14 years out of date. Why?
There is great interest in setting standards, and these standards are closely related to geopolitics. Every country is trying to develop its own standards. But there are exceptions, ISO and IEC standards basically cover all countries in the world. Medical electronic equipment is used more and more widely, and there are more and more ways of use, and various new technologies are also used in medical equipment in large quantities, which makes it difficult for relevant standards and regulations to complete the overall coverage of medical equipment. Equipment standards have lagged behind the development of medical equipment.
Many standards have their own specific directions, and medical device manufacturers should pay attention to the below standards:
ISO 13485 Medical Devices: Quality Management System (QMS) – Standard for Regulatory Purposes
ISO 13485 Medical Devices: A standard for regulatory purposes and is a quality management system (QMS) standard.
It is a standard for the medical machine industry that defines specific QSM requirements for the entire sample space. ISO 13485 is holistically designed for the design, production, installation and maintenance of medical devices. It also provides an auditing body that enables auditing by both internal and external parties.
ISO 14971 Medical Devices: Standard for Risk Management of Medical Devices
This standard was established to reduce and control the risks that medical products bring to patients. The ISO 14971 standard will continuously evaluate medical devices and evaluate the benefits and risks of medical devices.
The ISO14971 standard is aimed at the entire life cycle of medical equipment, and the whole process of design, production, use and maintenance of heavy medical equipment.
IEC 60601: Standard for Medical Electrical Equipment
IEC 60601 is a tribe of standards that are primarily aimed at electrical equipment. IEC standards cover electrical equipment performance and electrical system compatibility. IEC60601 includes more than 70 independent standards.
In the IEC60601 standard, medical electrical equipment is defined as electrical equipment that transmits energy to or receives energy from a patient. The IEC60601 standard deals with the basic safety requirements of medical equipment. Strict implementation of this standard can effectively reduce unacceptable risks to patients due to mechanical failures or functional defects.
FDA 21 CFR Part 807
Center for Devices and Radiological Health (CDRH) is a subset of the FDA, this standard is mainly for radioactive medical devices, and has a certain regulatory role for medical device companies selling in the United States. CDRH regulates all electronic products that emit radiation, not just medical equipment, such as microwave ovens, color TVs, sonars, etc.
FDA standards in the United States require that all organizations involved in the production and sale of medical devices must register with the FDA, including manufacturers, distributors, importers, and exporters.
FCC Title 47
This electronic equipment certification is related to Electromagnetic Interference (EMI). FCC certification is for all electronic devices that contain RF modules. Radio frequency will have a certain impact on human body functions. FCC certification has restricted radio frequency equipment, so as to avoid harm to human health.
Other important indicators of medical equipment
Safety is the most important factor for medical equipment, and various certifications are also regulated and restricted for the safety of medical equipment, which can greatly avoid medical accidents caused by equipment problems. In addition to safety, accuracy is an important indicator for measuring medical electronic equipment. The accuracy of medical equipment requires us to consider the performance of different materials and electronic devices when designing medical PCB, consider the quality of raw materials provided by suppliers when purchasing, and consider the impact of production processes on products when manufacturing.
In addition to accuracy, reliability and ease of use are two other important metrics. Reliability refers to the quality of medical electronic products. For the same type of medical products, the service life of products of different companies can vary greatly, which reflects the reliability of products of different companies. Reliable products are well-designed, using high-quality materials and components. Ease of use reflects a company’s product design capabilities. Products designed by companies with strong product capabilities are more in line with ergonomics and people’s usage habits, which can greatly reduce the cost and time for hospitals to learn and use.
Some Interesting Developments in Medical PCB

We talked about a lot of compliance and standards for medical PCBs before, let’s take a look at some of the latest advances in medical PCB.
Among all human senses, we attach the most importance to vision and hearing. Many of our medical devices are also developing in the direction of vision and hearing. The medical vision and hearing system plays a great role in the medical system. The latest scientists have made a great breakthrough in the human body’s sense of touch. A team at Stanford University is working on artificial skin, which is stretchable and has printed wiring with a large number of transistors. The skin also has different sensors that can sense changes in the environment.
Traditional gastroscopic monitoring is a very painful procedure, requiring a thin tube to pass through our throat and into the esophagus and stomach. The latest capsules with sensors allow patients to avoid this pain. The sensors in this capsule can determine the different gas and gas compositions in the stomach, which can provide strong support for doctors to diagnose patients. This technology is the cutting-edge technology of stomach diagnosis.
Conclusion
The development of science and technology is to improve human life, and science and technology exist to create a better life, which is best reflected in the development of medical technology. With the progress of medical treatment, the average life span of human beings has reached a high level. The use of various medical electronic devices has greatly increased the success rate of medical treatment. All kinds of disabilities and diseases can be effectively treated. Medical electronic equipment has increasingly become an indispensable part of medical treatment. The development of medical electronic equipment can not be separated from the progress of medical PCB technology. Breakthroughs in various advanced production processes can enable the production of medical PCB with different materials, which ensures the quality of medical electronic products and greatly reduces medical accidents.
MOKOMEDTECH is a medical PCB manufacturing company. We have a profound technical accumulation in the field of re medical PCB, and we are willing to contribute our strength to the progress of medical treatment.